93/206, Canal bank road,
Indira Nagar, Adyar,
Chennai – 600020


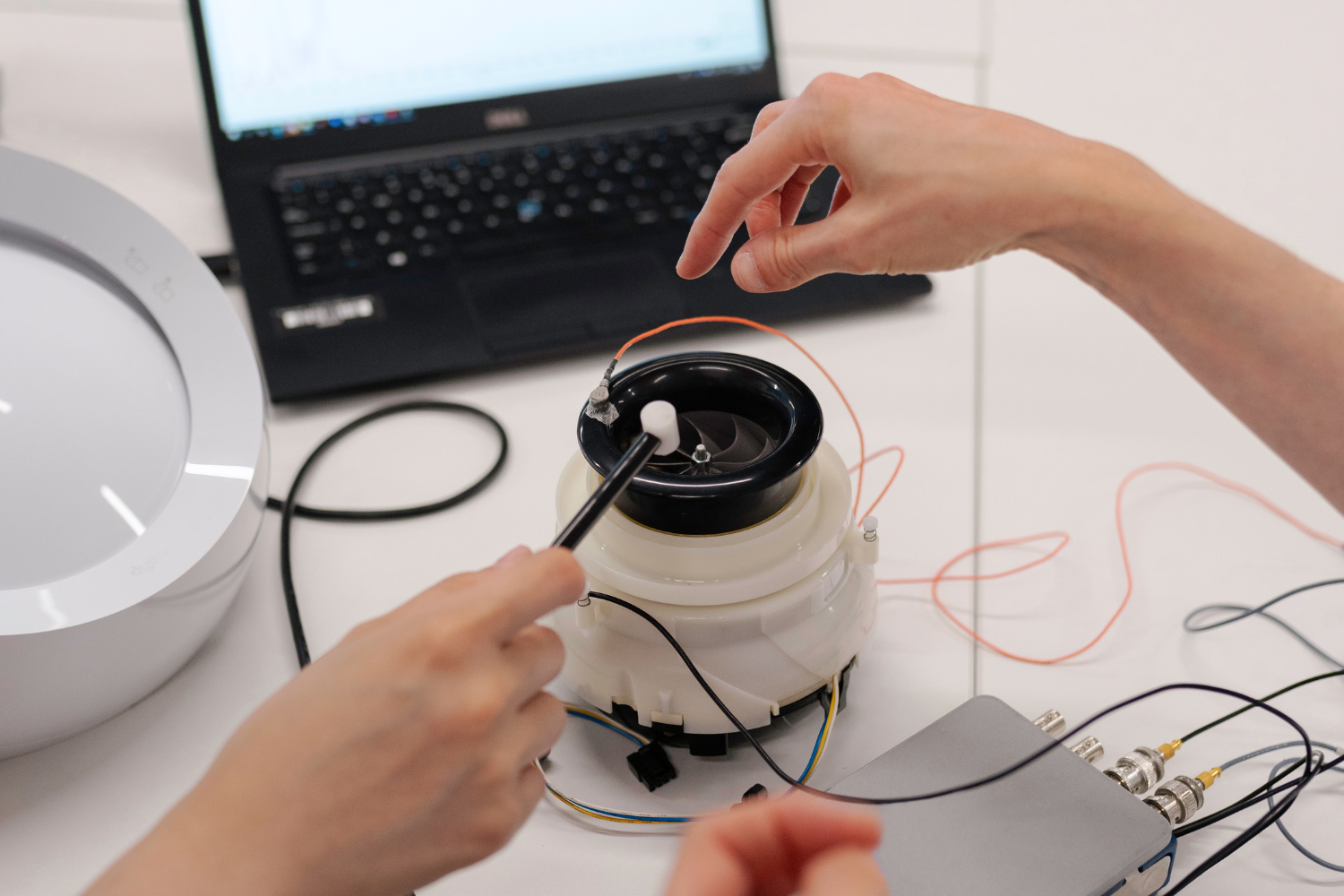
We conducted a thorough evaluation of the existing autoclave designs to pinpoint inefficiencies and areas for improvement. This granular assessment allowed us to understand the intricacies of thermal distribution, pressure dynamics, and material stress points.
Based on our findings, Clicky implemented a series of design enhancements aimed at maximizing performance. We provided updated mechanical drawings that integrated advanced engineering principles, making it easier for manufacturers to produce each autoclave with tighter tolerances and higher quality control.
We overhauled the BOMs to include parts that improved durability and functionality while reducing costs. Our revised manufacturing designs were optimized for efficiency, ensuring that production processes were both reliable and scalable. This method not only improved overall through but also reduced downtime and waste.
Prototypes were rigorously tested under various operational scenarios to guarantee that the new design met and exceeded industry standards for sterilization. Feedback from these tests was used to make fine adjustments, further enhancing performance reliability.